by David Harry & Jack McGurk, MPA
The complex battle to reduce healthcare-associated infections (HAIs) is being waged through campaigns for proper hand hygiene, understanding the dynamics of drug interactions, training to ensure PICC (Peripherally Inserted Central Catheter) lines are installed properly, actions to prevent surgical site infections, and ensuring proper environmental cleaning of hard surfaces. Evidence now suggests these, and many more, proactive approaches may be negatively impacted by unseen obstacles encountered during the cleaning and disinfecting process.
Floor care has gone through several paradigms during history to improve cleaning abilities. The first major shift came in June 1893, when Thomas W. Stewart of Detroit, Michigan, received his patent for the revolutionary string mop1, allowing personnel to mop floors from a standing position. This cleaning technique lasted for over a century until the second paradigm arrived with the introduction of the microfiber flat mop and its advantages in simplicity while utilizing less water and cleaning chemicals.2
Floors have been overlooked or discounted as a contributor of HAIs due to claims that they are rarely touched by patients. Monitoring high-risk objects (HROs) as a measure of cleaning efficiency by Carling and his colleagues did not include floor surfaces.3 In reality, floor surfaces have the potential to return to pre-disinfection bacterial levels within several hours after mopping.4 Pathogens are consistently introduced to the floor throughout the day by shoes, transport equipment such as wheelchairs and beds, treatment devices or computer carts, and non-slip patient socks that traverse the floors and frequently, directly into a bed. More importantly, there is a consistent potential for cross-contamination on and across the floor by an item expected to be clean and often handled without gloves, a freshly laundered mop.
It was estimated in a 2002 publication that 1.737 million HAIs occurred in hospitals in the United States that year resulting in 98,987 deaths, a rate of 5.8%.5 This highlights the seriousness of the problem facing healthcare facilities causing increased monitoring and reporting of HAIs by oversight agencies. There were 39 million patient stays in hospitals during 2009, with an average HAI cost of $1,024 per admission. The average HAI remedial cost per infected patient is therefore $23,735, accounting for a total HAI cost of $40.3 billion and representing 11% of total hospital spend in 20096. In addition, a recent report revealed that 769 hospitals are seeing a 1% cut in Medicare payments in the 2017 fiscal year for high rates of hospital-acquired conditions, as part of the HAC Reduction Program. For 241 of these facilities, this represents the third consecutive year of these penalties.7 The Centers for Medicare & Medicaid Services (CMS) penalties against 2,588 hospitals are expected to save Medicare $538 million during 2017, a direct cost, thus assumed, by those healthcare facilities in lost reimbursement.
Until recently, the challenge to floor hygiene has not been aggressive due to the lack of scientific evidence to support increasing cleaning levels and a corresponding reduction in HAIs. The landscape is changing as floors are being revisited with compelling, mounting evidence. During a survey of five Cleveland-area hospitals, researchers found floors in patient rooms to be frequently contaminated with HAI pathogens including Clostridium difficile (C. diff), found in 44% of rooms cultured after patient discharge cleaning and 53% with the patient housed in the room. Vancomycin-resistant enterococci (VRE) and methicillin-resistant Staphylococcus aureus (MRSA) were found present on the floors, although to lesser percentages. Additionally, of 100 occupied rooms, 41% had one or more high-touch objects such as blood pressure cuffs, pulse oximeters, heating pads, and bed linen in contact with the floor.8 This study demonstrates the potential sources of contamination present on the floors after discharge cleaning and with patient occupancy.
In an effort to reduce falls, especially among elderly patients, many hospitals have introduced the use of non-slip socks for patients. A recent report evaluated non-slip socks alongside floor samples. The study reported contamination of 85% VRE on the socks and 69% of floor samples. MRSA was found on 9% of socks and 17% of floor samples.9 The study indicated that the multidrug-resistant organisms, VRE (85%) and MRSA (9%), were found on socks that ambulatory patients wear when traversing their rooms or walking halls and serve as a potential mechanism to traffic these organisms throughout the hospital. To compound the problem, patients often wear non-slip socks to bed upon return to their room, consequently contaminating their beds and linens.
In 10 Arizona hospitals, a study to examine the effectiveness of laundering cloth and microfiber reusable towels used in cleaning and disinfection of rooms after terminal discharge of patients, revealed that viable microorganisms were found on 93% of the towels after laundering.10 The results of this study prompt similar inquiry whether parallel findings could be identified for reusable mops used to clean floors in patient care areas and throughout the hospital.
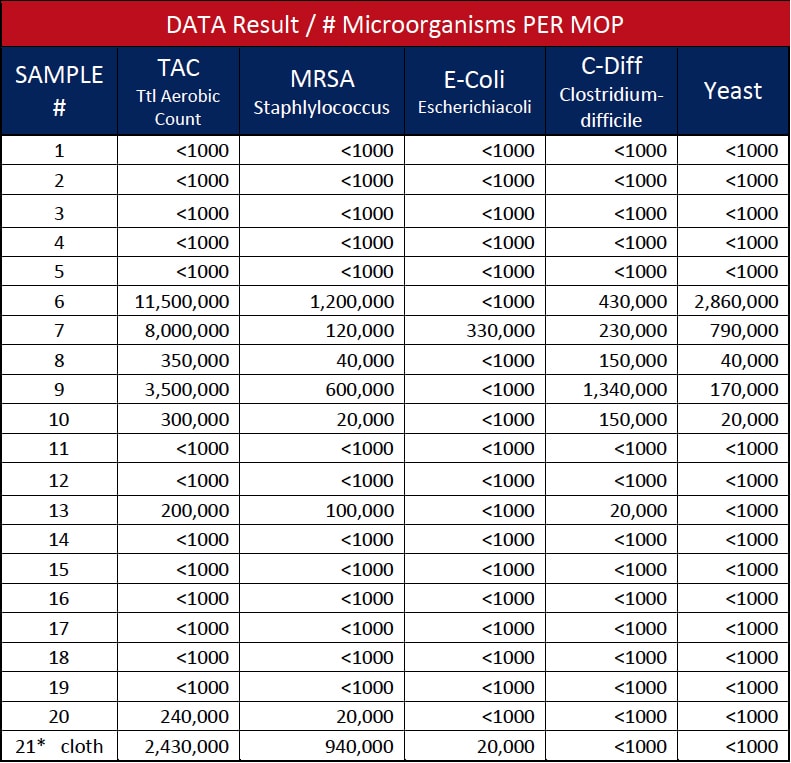
The authors gathered “clean”, newly laundered microfiber flat mops from 11 hospitals and had them tested for microorganisms. A total of 20 samples of newly laundered microfiber floor mops were collected from storage areas using latex gloves, immediately placing them into sterile plastic bags, then delivered to a third-party independent Laboratory for in vivo clinical testing.11
The modified standard plate count testing method consisted of placing each microfiber mop pad into a Ziplock plastic bag with 500 ml BPB. The bag was then placed into the Stomacher 400 Circulator and processed for three minutes at 250 revolutions per minute (rpm). The resulting diluent sample (10-1) was plated on designated selective media and incubated under specified conditions. A (10-2) dilution was also prepared and tested. Representative colonies recovered on the selective media were Gram stained for further verification.12
The microbiological tests on newly laundered flat mops resulted in seven mops (35%) with microbial contamination, as shown in Table 1. Three of the 11 hospitals’ mops were positive for pathogens, representing a 27.3% contamination rate as one of the hospitals was tested twice to confirm findings. The findings of HAI pathogens MRSA and C. diff confirms similar testing reported in the American Journal of Infection Control 2013 article.13 In effect, testing continues to increase awareness, combined with cited literature, to establish that laundered “clean” microfiber can, and likely is, contributing to cross-contamination within healthcare environments. Another important finding, shown as Sample 21 in Table 1, was that a microfiber cleaning cloth laundered with microfiber mops was found to contain HAI pathogens MRSA and C. diff. after the laundering was completed and the cleaning cloth was returned for use at the hospital. This confirms the potential cross-contamination of cloths from mops or vice versa through the laundering process.
It should be anticipated that removing biological contaminates from a microfiber textile would create more challenges than hard surfaced medical devices. In contrast, compelling sterile properties of single-use items are difficult to ignore when considering reprocessing, particularly to prevent the spread of infection or cross-contamination. HAIs are driving this market decision, especially when considering the complexity of textiles over and above basic equipment like scissors, tweezers and forceps. Textile reprocessing (laundering) is not a straightforward process because of the complex substrate, susceptible to residual chemical and biological contamination. Therefore, the laundry process, reprocessing agents (detergents), as well as material degradation, results in imperceptible damage and performance impairment that is impossible to manage. As a result, curtains, linens, towels and surgical garments are now available as single-use items.
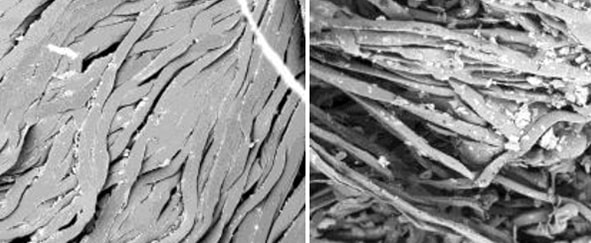
The typical hot water temperature in the laundering process specifies 160oF – 200oF for microfiber mops or cloths and excludes the use of bleach unless necessary. Bleach is required for a cold-water laundering method at 5-150 ppm. Any organic components in laundered items negate the sanitizing properties of bleach as shown in Figures 2 and 3. Fabric softeners can also adversely impact the laundering efficacy of microfiber products in similar fashion, due to their ability to attach to the microfiber and impact its structure and performance. Finally, drying microfiber above a (low) heat setting of 130oF inhibits the retention qualities of the mop or wipe, due to heat damage, or melting of the microfiber itself as shown in Figure 1, photograph 3. The complex process for microfiber laundering is extensive, with varying manufacturers’ recommendations dependent on the quality and source of microfiber. Compounding the above-referenced factors is that the laundry facility protocol must be strictly implemented, controlled and updated with each new microfiber mop sourced.


Many hospitals are moving to single-use microfiber mops and wipes to help reduce the risk of HAIs from floor surfaces. Single-use mops remove the potential of cross-contamination with virgin microfiber in every use, while eliminating the risk of efficacy degradation through microfiber structural breakdown or pathogen retainage in the mop, as a result of an inadequate laundering process.
Environmentally-conscious hospitals will evaluate using single-use microfiber mops and the corresponding waste implications. Factors to consider include single-use microfiber mops using less water and energy by eliminating the laundering cycle. Furthermore, in a fully occupied 500-room hospital, daily single-use mop waste would equate to 39.6 pounds using two mops per room (~ 36 grams per room). This represents a 0.25% increase in waste generation, a nominal impact when each staffed bed produces an average of 33.8 pounds of daily waste, or 16,900 pounds for the hospital as a whole.17
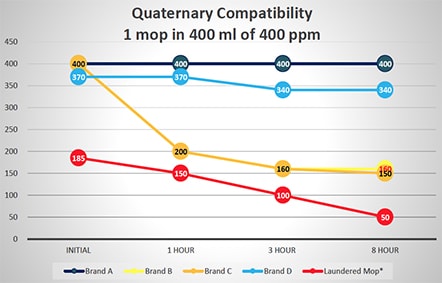
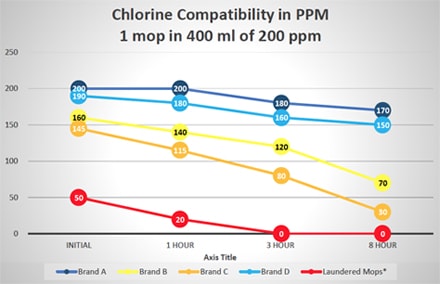
Additional tests were run to evaluate the potential of disinfectant neutralization on several brands of single-use and laundered microfiber mops. These tests reflect square feet of floor coverage achieved by the microfiber mops, based on their ability to absorb liquid from a charging container and liquid released from the mop to the floor. Figure 2 graphically displays this data from testing a single mop in 400 ml of 400 ppm quaternary ammonium disinfectant over an eight-hour period. Similar testing was done using a 400-ml solution of 200 ppm chlorine disinfectant with results shown in Figure 3. As shown in Figures 2 and 3, there is a variance in how single-use mops neutralize either the quaternary ammonium and chlorine disinfectants. Some single-use brands are more efficacious, maintaining disinfectant capability, which allows them to deliver the disinfectants to the floor. While all single-use brands performed better than the laundered mops in base testing, residual laundering residue on mops will neutralize the disinfectants even more rapidly. Based on the additional solution required, the laundered mops performed worse than the testing indicated since 500 ml of quaternary ammonium and chlorine solution was required to saturate the mops and provide enough solution for titrations
Variability of Several Brands of Single-use and Laundered Microfiber Mops in Neutralizing Quaternary Ammonium Disinfectant
(500 ml of Quat Required for Laundered Mop to Provide Enough Solution for Testing)
Variability of Several Brands of Single-Use and Laundered Microfiber Mops in Neutralizing Chlorine Disinfectant
(500 ml of Quat Required for Laundered Mop to Provide Enough Solution for Testing)
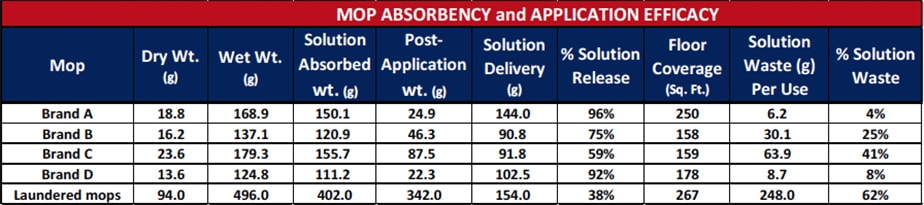
Further testing was performed on commercially-available, single-use microfiber mop brands and laundered reusable microfiber mops to determine the absorbency, application efficacy, and quantity of wasted disinfectant when cleaning, as shown in Table 2. Mops were weighed in a dry state, allowed to absorb as much fluid as possible from a charging container, then weighed in the wet state. Dry and wet weights were recorded for each brand of microfiber mop. Each mop was applied until the solution was fully dispensed onto the floor. Square footage of the area mopped was measured and recorded for each brand. After completing this test, each mop was again weighed to determine the amount of disinfectant delivered to the floor and how much was retained on the mop. The remaining mop solution is ultimately lost to the waste stream during disposal or reintroduced into the laundry system. The results of this testing are shown in Table 2. There was a great deal of variation between four brands of single-use mops with only one able to cover an average hospital room size of 250 square feet.
Test Results for Several Microfiber Mop Brands for Absorption, Application Efficacy, and Wasted Disinfectant Product
Floors, often overlooked in the past as a major factor of environmental contamination leading to increased HAI rates, are, in fact, a contributor to this very expensive and life-impacting problem. Studies have shown that floors harbor HAI pathogen organisms. These pathogens may not be neutralized by using mops that bind disinfectants or may be transported through unexpected means, including socks or laundered mops damaged by the laundering process and reducing their ability to effectively clean or disinfect the floor.
Inadequately laundered mops can be reintroduced to the hospital with remnant HAI pathogens. This study evaluated laundered microfiber mops from 11 hospitals and found that 27.3% of the newly laundered mops contained microbial contamination, including HAI pathogens. To reduce HAI risk exposure, hospitals should convert to single-use microfiber mops. This study evaluated several brands of single-use microfiber mops and determined that differences vividly exist between brands on several critical criteria such as disinfectant neutralization, absorbency and dispersion/release efficacy, floor coverage, and wasted chemical solution. All of these factors need to be evaluated in determining which single-use product to pursue as an added solution in reducing HAI exposure, while minimizing environmental impact and achieving the greatest efficacy in the cleaning/disinfecting process.
1. https://www.google.com/patents/us499402
2. US EPA: Using Microfiber Mops in Hospitals, Environmental Best Practices for Health Care Facilities, November 2002: https://www3.epa.gov/region9/waste/p2/projects/hospital/mops.pdf
3. Carling PC, Parry MF, Von Beheren SM. Identifying opportunities to enhance environmental cleaning in 23 acute care hospitals. Infect Con Hosp Epi 2008; 29:1-7.
4. Ayliffe GAJ, Collins DM, Lowbury EJL. Cleaning and disinfection of hospital floors. Br. Med J 1966: 2:442-5.
5. Klevens RM, Horan TC, Gaynes RP, Pollock DA, Cardo DM. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Pub Health Reports March-April 2007: vol 122:160-164.
6. R. Douglas Scott II, Economist. The direct medical costs of healthcare-associated infections in U.S. hospitals and the benefits of prevention, Division of Healthcare Quality Promotion, National Center for Preparedness, Detection, and Control of Infectious Diseases Coordinating Center for Disease Control and Prevention, March 2009.
7. Becker’s Infection Control & Clinical Quality: The 241 hospitals punished 3 years in a row for high infection rates, December 27, 2016: http://www.beckershospitalreview.com/quality/the-241-hospitals-punished-3-years-in-a-row-for-high-infection-rates.html
8. Deshpande A, CadnuJL, Fertelli D, et al. Are hospital floors an underappreciated reservoir for transmission of health care-associated pathogens? American Journal of Inf. Control. 45, 2017: 336-338.
9. Mahida N, Boswell T. Non-slip socks: a potential reservoir for transmitting multidrug-resistant organisms in hospitals? J Hosp Infect 2016; 94: 273-5.
10. Sifuentes LY, Gerba CP, Weart I, Engelbrecht K, Koenig DW. Microbial contamination of hospital reusable cleaning towels. American Journal of Infection Control xxx 2013: 1-4.
11. The studies were conducted in compliance with the principles of Good Laboratory Practice (GLP) 21 CFR Part 58: Good Clinical Practice (GCP) 21 CFR Parts 50, 54, 56, 312, 314 and ICH Guidelines Good Clinical Practice (E6)
12. Ibid.
13. Sifuentes LY, op. cit.
14. Fireman Z. Biopsy forceps: reusable or disposable? J Gastroenterol Hepatol. 2006 Jul: 21 (7):1089-92
15. Sbutega-Milosević G, Slepĕvić V, Marmut Z, Bujko M, “Importance of disposable medical materials and instruments in the prevention of in-trahospital infections”. (Article ins Serbian) Vojnosanit Pregl. 2000 Jan-Feb: 57 (1):55-8
16. Diab-Elschahawi M, Assadian O, Blackly A, Stadler M, Pernicka E, Berger J, et al. “Evaluation of the decontamination efficacy of new and reprocessed microfiber cleaning cloth compared with other commonly used cleaning cloths in the hospital” American Journal of Infection Control, May 2010; 38:289-92
17. Rastogi N, Wasting Syndrome-How much trash do hospitals produce? The Green Lantern, Illuminating Answers to Environmental Questions, Oct. 19, 2010 (June 15, 2017) www.slate.com/articles/health_and_science/the_green_lantern/2010/10/wasting_syndrome.html

Providing proper hand hygiene systems for your employees and guests has never been more vital...

Hospital Grade One-Step Cleaner Wipes BCI is Now Offering Diversey™ Avert® Sporicidal Disinfectant Cleaner Wipes...

Providing proper hand hygiene systems for your employees and guests has never been more vital...

Hospital Grade One-Step Cleaner Wipes BCI is Now Offering Diversey™ Avert® Sporicidal Disinfectant Cleaner Wipes...















